If you’ve ever worked with plumbing, roofing, or any project involving metal, you’ve probably heard warnings about mixing copper and galvanized steel. But why does this combination cause problems? Let me walk you through everything I’ve learned about the copper and galvanized steel reaction—why it happens, where it’s likely to occur, and how to prevent costly damage.
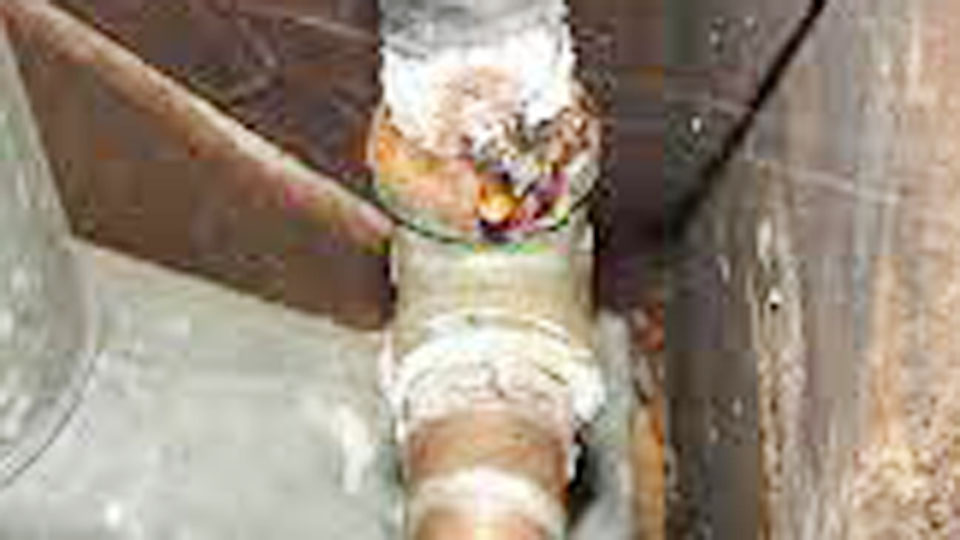
Image by klp99mall
If you’re a homeowner, DIY enthusiast, or professional, understanding this interaction can save you time, money, and headaches.
What Happens When Copper Meets Galvanized Steel?
Copper and galvanized steel are both popular materials, but they don’t play well together. When these two metals come into contact in the presence of an electrolyte (like water), a chemical reaction called galvanic corrosion occurs.
This reaction happens because copper and zinc (the coating on galvanized steel) have different electrochemical properties. Copper acts as a “noble” metal, while zinc is more “active.” When connected, zinc corrodes faster than it normally would to protect the copper.
Think of it like a battery: the metals create an electrical circuit, and the zinc sacrifices itself to keep the copper intact. Over time, this leads to pitting, leaks, or even structural failure in galvanized steel pipes or fittings.
Breaking Down the Science Behind Galvanic Corrosion
To grasp why copper and galvanized steel react, let’s dive into the basics of galvanic corrosion. All metals have a natural electrical potential, which is their tendency to lose electrons.
When two dissimilar metals touch in a conductive environment (like rainwater or humidity), electrons flow from the more active metal (zinc) to the less active one (copper). This exchange accelerates the corrosion of the active metal.
Here’s a simple analogy: imagine two runners in a race. The zinc-coated steel is sprinting to protect the copper, but it tires out quickly, leaving the steel vulnerable. Without the zinc coating, the steel underneath rusts rapidly.
Common Places Where Copper and Galvanized Steel Interact
You might not realize it, but copper and galvanized steel are often used in close proximity. Here are a few scenarios where their interaction becomes a problem:
- Plumbing Systems: Older homes might have copper pipes connected to galvanized steel pipes. Even a small section of copper can trigger corrosion in the steel over time.
- Roofing and Gutters: Copper flashing or decorations near galvanized steel gutters can lead to rust streaks or leaks.
- HVAC Systems: Condensation or moisture in heating/cooling systems can create the perfect environment for galvanic corrosion.
- Marine Environments: Boats or docks using both metals in saltwater face accelerated deterioration.
Consequences of Ignoring the Reaction
Ignoring the copper-galvanized steel reaction can lead to serious issues:
- Pipe Leaks: Corroded galvanized pipes may develop pinhole leaks, causing water damage or mold.
- Reduced Lifespan: Galvanized steel components might fail years earlier than expected.
- Costly Repairs: Replacing rusted pipes, gutters, or structural parts is expensive and disruptive.
- Safety Risks: In extreme cases, corroded supports or fittings could collapse.
I’ve seen homes where a single copper fitting caused thousands of dollars in plumbing repairs. The damage starts small but snowballs quickly.
How to Prevent Galvanic Corrosion Between Copper and Galvanized Steel
EZ-Fluid 3/4 Inch Dielectric Union Sweat Solder x FIP,Brass Solder Connection, Female Threaded Galvanized Steel Body,Reduces Electrolysis,Use as Water Heater Dielectric Unions (1)
- EZ-Fluid Dielectric Union Sweat Solder x FIP ,Brass Solder Connection, Female Threaded Galvanized Steel Body,Reduces Electrolysis ,Use as Water Heater Dielectric Unions and More
- COMPATIBILITY: Designed for connecting dissimilar metal pipes, particularly copper to steel or galvanized piping systems Size Available from 1/2″ to 2″
- INSULATION: Effectively separates different metals to prevent galvanic corrosion while maintaining a watertight seal
- QUALITY- Designed to withstand a maximum pressure of 250 PSI and the maximum temperature for safe use is 180-degrees Fahrenheit. The IPS connection and nut are both constructed of steel.
- DIMENSIONS: Standard size fitting with brass ring diameter matching common residential and commercial plumbing specifications
The good news? You can prevent this reaction with a few practical steps:
Use Dielectric Unions
These fittings have a plastic or rubber sleeve that separates copper and galvanized steel, blocking electrical contact. They’re a must-have in plumbing systems where the two metals meet.
Apply Protective Coatings
Paint or epoxy coatings create a barrier between the metals. Just ensure the coating remains intact and free of scratches.
Replace One of the Metals
If possible, use all-copper or all-galvanized steel systems. For example, replace a section of galvanized pipe with PVC or CPVC to avoid direct contact.
Install Sacrificial Anodes
Zinc or magnesium anodes can be attached to galvanized steel. These “sacrifice” themselves by corroding first, protecting the steel.
Keep the Area Dry
Since moisture fuels galvanic corrosion, fix leaks promptly and use waterproof insulation in damp areas.
Comparison of Copper vs Galvanized Steel
| Property | Copper | Galvanized Steel |
|---|---|---|
| Corrosion Resistance | High (resists rust) | Moderate (zinc coating wears off) |
| Electrical Conductivity | Excellent | Low |
| Cost | Expensive | Affordable |
| Common Uses | Plumbing, wiring, roofing | Fencing, pipes, structural frames |
| Reactivity | Less active (noble) | More active (sacrificial) |
Fiberglass Repair Kit, 3 x 132 In, Gray
- Adhesives and Sealants
- Package Dimensions: 17.526 H x 4.572 L x 9.144 W (centimetres)
- Package Weight: 0.204 kilograms
- Country of Origin : China
Real-Life Examples of Copper-Galvanized Steel Reactions
Let me share a story from my own experience. A friend once called me about a mysterious leak under their kitchen sink. They’d recently installed a copper faucet connected to old galvanized pipes. Within months, the joint where the metals met had corroded badly, causing a slow drip. We fixed it by adding a dielectric union and replacing the damaged pipe section.
Another example: a coastal restaurant used copper decor on its galvanized steel railing. Saltwater spray accelerated the corrosion, leaving unsightly rust stains. They solved it by switching to stainless steel brackets and applying anti-corrosion paint.
Maintenance Tips for Existing Systems
If your home or project already uses copper and galvanized steel together, here’s how to maintain it:
- Inspect Regularly: Check for white rust (zinc oxide), discoloration, or flaking around joints.
- Test Water Quality: High mineral content in water can speed up corrosion. Use a water softener if needed.
- Clean Surfaces: Remove dirt or debris that might trap moisture against the metals.
- Monitor pH Levels: Acidic environments (pH below 7) increase corrosion rates.
Wrapping It Up
Understanding the reaction between copper and galvanized steel is crucial for anyone working with these metals.
By recognizing where they interact, using preventive measures like dielectric unions, and staying vigilant with maintenance, you can extend the life of your systems and avoid unexpected repairs.
Galvanic corrosion doesn’t happen overnight—it’s a slow process that’s easy to overlook until it’s too late. Take action now to protect your investments and keep everything running smoothly.
FAQs About Copper and Galvanized Steel Reactions
Can copper and galvanized steel touch directly?
No, direct contact in the presence of moisture will trigger galvanic corrosion. Always use a dielectric union or insulator.
How long does it take for corrosion to occur?
It depends on environmental factors, but damage can appear within months in humid or wet conditions.
Can I paint over the metals to prevent a reaction?
Yes, but the coating must be thick, non-conductive, and regularly maintained to avoid gaps.
Is stainless steel compatible with copper?
Stainless steel is less reactive than galvanized steel but can still corrode when paired with copper. Use dielectric fittings as a precaution.
What if I already have copper and galvanized steel connected?
Install a dielectric union immediately and inspect the system for existing damage. Replace corroded parts as needed.
Can I use Teflon tape to separate the metals?
Teflon tape isn’t sufficient—it degrades over time. Opt for dielectric unions or specialized coatings instead.
Does galvanic corrosion affect drinking water?
Yes, corroded pipes can leach zinc or iron into water, affecting taste and safety. Replace damaged pipes promptly.
By staying informed and proactive, you can master the challenges of working with copper and galvanized steel. Feel free to reach out if you have more questions—I’m here to help!

